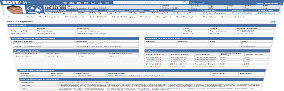
Innovative and automated features and functionality designed to enhance the CAR-T pre-infusion workflow,
including the Co-Morbidity Index, Selection Conference tool, and the HCT Scheduler and Calendar
Co-Morbidity Index
Identify and understand the pre-infusion comorbidities that could potentially lead to post-CAR-T infusion complications, including toxicities.
The HCT-Comorbidity Index automatically calculates an Index Total Score based on Medical, Cardiovascular, and Psychiatric conditions, as well as prior preventive maintenance, malignancies, and surgeries.
A summary of problems and complications is provided along with pertinent negatives.
Selection Conference Tool
The key criteria data needed to determine eligibility for CAR-T therapy is right where it needs to be… at your fingertips.
An auto-generated Selection Conference Report provides information such as the confirmed diagnosis; results of previous chemotherapy treatments, and status of autologous stem cell transplant, as well as organ, cardiac, and pulmonary function measures.
Conference decisions and other information is captured and can be sent to the EHR as an outbound note.
HCT Scheduler/Calendar
If overbooking patients for infusion is an issue, EDITLife’s HCT Scheduler and Conditioning Calendar are the solution.
As soon as you choose a conditioning regimen, the Scheduler alerts you of resource conflicts and, if necessary, provides the next best date for infusion.
An auto-updated Conditioning Calendar shows when patients are scheduled for each step in the process – by patient, day, week, or month.
Interconnected
Product Collection
and Processing Suite
A series of interconnected screens that “talk” to each other – data entered on one screen is auto-updated in the appropriate fields in screens throughout the application.
EDITLife’s Collection and Processing Suite is a series of interconnected screens – Source Selection, Mobilization, and Collection – that walk you through each step.
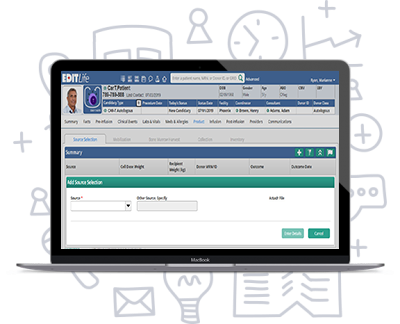
Source Selection
Choose the source (e.g., Peripheral Blood, Bone Marrow Aspirate, Byoptic Sample), plan and track collection outcomes, document recipient information, such as height and weight.
Mobilization
Track start and end dates, indicate central line placement, and identify growth or mobilizing factors – document mobilization data, including medication name and strength.
EDITLife automatically displays labs and graphs (with medications if desired) on the same screen – all without IT assistance and without jumping to another system.
Collection
Summary and detailed information related to the processed collection – capture, e.g., product codes, ISBT codes, absolute cell counts, contamination data, product distribution and disposition information, and product shipping details.
Cell Therapy Product tracking includes the manufacturer, as well as product processing, manipulation, analysis, cell count, and disposition data.
Innovative Post-CAR-T Infusion Features
EDITLife enhances your ability to monitor the ever-expanding group of CAR-T patients' outcomes post-infusion and to comply with FDA standards.
The system provides the tools needed for following patients for the FDA-required period of 15 years to assess the risk of development of subsequent neoplasms and other potential adverse effects.

INDICATION STATUS
Track primary indication Status post-infusion for patient care and reporting.
Auto-generated summary of the primary indication displays Treatments and Procedures as well as Status information (First Remission, Second Remission, Relapse, etc.)
TOXICITY ASSESSMENT
Monitor and manage the unique toxicities associated with CAR-T therapy.
Streamlined methodology for capturing and summarizing Assessment and Treatment data for adverse events, including Cytokine Release Syndrome (CRS) and Neurotoxicity.
PRODUCT PERSISTENCE
Evaluate the persistence of CAR-T cells post-infusion.
Track the evaluation method (Molecular Assay, Flow Cytometry, Immunohistochemistry, Other), cell source, and infused cell detection over time.
Auto-populate and electronically submit CAR-T forms
supported by CIBMTR for electronic submission
The EDITLife team works closely with CIBMTR to validate and verify the electronic submission
of newly created CAR-T forms and HCT forms modified to also collect CAR-T data.
Everything you need for CIBMTR CAR-T forms in one place
Check out EDITLife today!
See for yourself how EDITLife tracks CAR-T patient outcomes and simplifies CIBMTR CAR-T form reporting.
REQUEST A DEMO NOW!